Submitted by itadmin on
The Basque Country and Réunion Island, an overseas department of France in the Indian Ocean, have the world’s highest prevalence of type 2A limb-girdle muscular dystrophy. This hereditary pathology, classified as a rare disease, is also known as calpainopathy. Its high prevalence in the Basque Country can be explained by a mutation typical to the territory, popularly known as the Basque mutation, resulting from the high level of endogamy that has existed in that region for decades.
The Molecular Diagnostics Platform of the Biodonostia Health Research Institute, which forms part of Biocores, is the reference center for the diagnosis of limb-girdle muscular dystrophy. Department head Pilar Camaño states that the center has developed a consolidated line of research on calpain. Having studied the pathology over many years has given the team a rich experience in this type of dystrophy.

Image: Biodonostia Health Research Institute
Reference center for neuromuscular diseases
Limb-girdle muscular dystrophy is not the only rare disease that this staff of Basque scientists is working on. The Molecular Diagnostics Platform, created in 2014 from a group led by neuromuscular pathology expert Adolfo López de Munain, offers a varied service portfolio in the detection of the molecular defect of disorders that occur with frontotemporal dementia, Parkinson’s disease or different types of muscular dystrophies and myopathies. The Platform is also a national reference center for the diagnosis of facioscapulohumeral muscular dystrophy.
According to data from Orphanet, this second rare neuromuscular pathology occurs in one out of every 20,000 people and is characterized by a progressive course that affects skeletal muscle, without compromising any other organs. “From 2000 up to the present we have studied some 3,000 patients referred to us from nearly every province of Spain. Samples come in from public as well as private hospitals,” says Pilar Camaño in remarks to Biocores. In recent months, her Department has also received samples from Istanbul (Turkey) and Antwerp (Belgium).
Camaño, head of the Biodonostia department, specialized in molecular diagnosis and research of facioscapulohumeral muscular dystrophy type 1 (FSHD1) in the late 1990’s. The European reference group on this neuromuscular pathology is based in Leiden (Netherlands), where she worked to learn various techniques applied to its diagnosis, most notably pulsed-field electrophoresis and the study of methylation. Since then, comprehension of this dystrophy has increased markedly thanks to the technological cores.
Two types of facioscapulohumeral dystrophy... for now
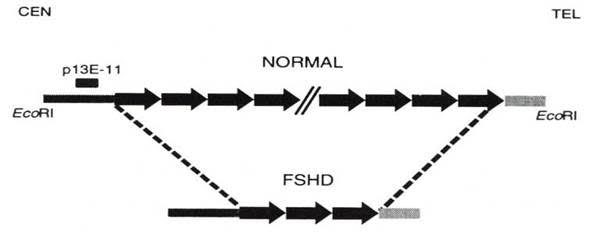
“In this disease, there occurs a reordering in the long arm of chromosome 4 (locus 4q35) in which there is an EcoRI polymorphic fragment made up of a number of 3.3 kb tandem repeats (d4Z4),” explains Pilar Camaño. “In the control population this fragment is greater than 38 kb (10 repeats) and patients with FSHD1 have less than 38 kb. Normally, the fewer repeats you have, the worse the clinical condition,” Camaño adds.
Image provided by Pilar Camaño (IIS Biodonostia)
“These patients have hypomethylation in this repeat region (4q35), which was caused by the deletion of repeats,” says the researcher. “It was also known that inside each of these repeats there was a gene called DUX4, but at first, expression of this gene had not been detected. In a normal state, this DUX4 gene is repressed, methylated. It is not expressed.” Camaño clarifies.
In 2010 it was found that FSHD1 patients, in addition to a deletion of D4Z4 repeats, also had to have a permissive haplotype (certain polymorphisms found after the repeats made this DUX4 gene from the last repeats create a polyadenylation signal that led the DUX4 gene to express itself. That expression was toxic for the muscle). “You can have a deletion of repeats but not a permissive haplotype. In that case this gene is not expressed and the person does not develop this disease,” says the head of the Biodonostia Molecular Diagnostics Platform.
Nevertheless, researchers observed that, in some cases that seemed to have a clinical picture typical of FSHD, the patients were negative for facioscapulohumeral muscular dystrophy type 1 (approximately 5% of cases). Two years later, it was found that this group of patients had mutations in a different gene (SMCHD1), located on chromosome 18. This gene is in physical contact with the D4Z4 repeats, repressing expression of the DUX4 gene. When there are mutations in the SMCHD1 gene, the protein does not function properly, and stops repressing the DUX4 gene of the last repeat, which, in the presence of a permissive haplotype allows it to be expressed. This is toxic for the muscle.
Image: Pixabay
“Patients with mutations in the SMCHD1 gene, who were called FSHD type 2, also have hypomethylation in the chromosome 4 region, which is also more marked than in cases of FSHD type 1,” Pilar Camaño tells Biocores. According to the specialist, “There are still cases that, even though they meet all the ‘requisites’, are neither FSHD type 1 nor type 2,”. She believes that a third type of facioscapulohumeral muscular dystrophy may be found in the future.
The Molecular Diagnostics Platform of the Biodonostia Health Research Institute benefits from the service offered by its own Genomics Platform. To create gene panels of neuromuscular pathologies and other disorders that require massive sequencing, the group works with the PGM Ion Torrent and in the case of the Sanger sequencing with an ABI Prism 3130. On another note, in cases of FSHD, Camaño clarifies that the protocol is different, and much more complex. “We have to digest the DNA, do horizontal or pulsed-field electrophoresis, perform Southern blots, radioactive labeling (phosphorous-32 isotope), do hybridization probes and autoradiography,” she explains. All of these make up a broad variety of techniques that, in recent years, have given scientists a better understanding of what is at the root of a health problem like rare neuromuscular dystrophies.