Submitted by itadmin on
When Erwin Schrödinger received the Nobel Prize in Physics in 1933, he said “it is fair to state that we are not going to experiment with single particles any more than we will raise dinosaurs in the zoo”. Last year, Stefan W. Hell recalled this remark when he received the Nobel Prize in Chemistry for the development of nanoscopy, an award he shared with scientists Eric Betzig and William E. Moerner.
During his speech at the banquet, Hell joked that “as a Nobel Laureate you should say ‘this or that is never going to happen’” as this would increase your chances of being remembered decades later at a similar ceremony. Yet aside from the tale, Schrödinger’s comment revealed the enormous scientific achievement made by nanoscopy. This technology has overcome the diffraction limit of light, a milestone that Timo Zimmermann, Head of the Advanced Light Microscopy Unit at the Centre for Genomic Regulation (CRG), says has “improved our understanding of biology” since we can now see the internal structure of a cell at unprecedented detail or view the components of a synapse between two neurons and see how little dendritic structures change their morphology during neural activity. These new nanoscopy techniques are based on light microscopy, a technique that, according to Stefan W. Hell, who attended Lindau Nobel Laureate Meeting, “allows us to observe the inner cell with minimal invasion and is used in most of the studies in cell biology (near 80%)”.
However, achieving this scientific goal has not been easy. For decades, the diffraction limit of light seemed like an insurmountable barrier since it was not possible to resolve image details below approx. 200 nanometres. Today, superresolution has enabled us to go down to the nanomolecular scale at around 20 nm. But how did we get here?
Zimmermann explains that there are two ways to overcome what is also known as Abbe’s limit. Firstly, Eric Betzig and William Moerner’s method, known as “single-molecule microscopy”, relied upon the possibility to turn the fluorescence of individual molecules on and off. In other words, as Hell described in his Banquet speech, this first method consisted of “calling on each molecule individually”.
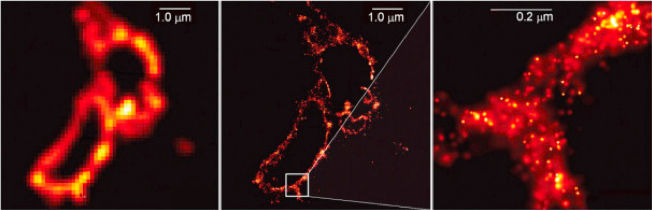
When explaining his trick, Hell once again joked that he never had that kind of patience to call on each molecule one by one: “I just told all of them to be quiet, except for a selected few that should speak up”. What Hell described was the stimulated emission depletion (STED) microscopy. In this case, “two laser beams are used: one stimulates fluorescent molecules to glow, another cancels out all fluorescence except for that in a nanometre-sized volume”. In the Lindau Nobel Laureate Meeting, Hell described this method explaining that “the resolution limiting effect of diffraction can be overcome by fully exploiting the properties of the fluorophores”. In other words, “scanning over the sample, nanometre by nanometre, yields an image with a resolution better than Abbe’s limit”, as the Nobel Prize Foundation explained in the press release. In 2005, Hell showed that nanoscopy made it possible to observe lysosomes like never before:
Ten years after the publication of those photographs, what progress has been made by nanoscopy? As Zimmermann points out, “this technique has allowed us to see live and in greater detail structures such as synaptic vesicles, nuclear pores and also ciliar structures, which play a key role in cell movement and hearing mechanisms”. The head of the CRG’s technical facility also compares nanoscopy with the “need to wear glasses”, since it helps us to see in greater detail and specificity biological structures which before had seemed blurred due to Abbe’s diffraction limit.
A practical application of superresolution microscopy is, for example, in the study of microorganisms such as bacteria or yeast. Although previously we could see these living beings, nanoscopy helps us resolve their cellular organisation in greater detail, thus improving our understanding of the mechanisms of pathogenicity of some of these organisms. Nanoscopy may even be crucial in learning more about genomic regulation, as demonstrated by recent research by the Centre for Genomic Regulation and the Institute of Photonic Sciences (ICFO) published in Cell.
There is no doubt that the future of this technology is very promising. As Zimmermann puts it, “e.g. the possible combination of nanoscopy with light-sheet microscopy may be a very interesting field for future developments”. Although we cannot predict what we will be able to observe with nanoscopy, “without the ability to see something in relation to its environment, we will never understand it”, says the CRG researcher.
Zimmermann argues that better visualisation of biological structures will also highlight “errors” during the sample treatment more clearly. Hence labelling will need to be further improved in the coming years. Another future challenge lies in the use of different types of light, since wavelength can alter cell viability. Here there are scientific groups working on the application of red or infrared light in order to damage cells as little as possible. These challenges were examined at the recent European Light Microscopy Initiative (ELMI 2015), a conference held in Sitges, also to discuss the future of nanoscopy. It is a future in which, as Schrödinger predicted, we will not be able to raise dinosaurs (at least for now), but where we will be able to experiment with individual particles to better understand the world around us.
Images from: Nobel Prize Foundation - Popular Information (Nobel Prize in Chemistry 2014)